There are Millions of chemical compounds everywhere around us. So one question can come in our mind that How can we Identify these compounds?
Spectroscopy is the answer of this question. There are a number of spectroscopy techniques by which we can easily know about the structure of the compounds, which elements are present in the compound. In other words we can say that spectroscopy is finger-prints of chemical compounds. By fingerprints we can distinguish between two humans, in the same way the compounds can be distinguished from others.
Spectroscopy, study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. More recently, the definition has been expanded to include the study of the interactions between particles such as electrons, protons, and ions, as well as their interaction with other particles as a function of their collision energy. Spectroscopic analysis has been crucial in the development of the most fundamental theories in physics, including quantum mechanics, the special and general theories of relativity, and quantum electrodynamics. Spectroscopy, as applied to high-energy collisions, has been a key tool in developing scientific understanding not only of the electromagnetic force but also of the strong and weak nuclear forces.
Spectroscopic techniques have been applied in virtually all technical fields of science and technology. Radio-frequency spectroscopy of nuclei in a magnetic field has been employed in a medical technique called magnetic resonance imaging (MRI) to visualize the internal soft tissue of the body with unprecedented resolution. Microwave spectroscopy was used to discover the so-called three-degree black-body radiation, the remnant of the big bang (i.e., the primeval explosion) from which the universe is thought to have originated. The internal structure of the proton and neutron and the state of the early universe up to the first thousandth of a second of its existence are being unraveled with spectroscopic techniques using high-energy particle accelerators.
Quantization of Energy
The meaning of quantization is that the energy is discreet. Our sample (compound) will absorb a particular type of energy, if we apply other type of energy then the sample will not absorb it. Here are some examples to understand this concept better.
Radio-waves are absorbed by the Nucleus of the atom
Infra-red waves are absorbed by the bonds of the samples and is related with vibrational energy.
Microwave energy is absorbed by gaseous samples and is related with rotational energy.
Ultraviolet- visible energy is absorbed by the electrons present in the sample and is related with electronic energy.
X-ray energy is approximately equal to the Ionization of core electrons.
General Principles
One of the central concepts in spectroscopy is a resonance and its corresponding resonant frequency. Resonances were first characterized in mechanical systems such as pendulums. Mechanical systems that vibrate or oscillate will experience large amplitude oscillations when they are driven at their resonant frequency. A plot of amplitude vs. excitation frequency will have a peak centered at the resonance frequency. This plot is one type of spectrum, with the peak often referred to as a spectral line, and most spectral lines have a similar appearance.
Spectra of atoms and molecules often consist of a series of spectral lines, each one representing a resonance between two different quantum states. The explanation of these series, and the spectral patterns associated with them, were one of the experimental enigmas that drove the development and acceptance of quantum mechanics. The hydrogen spectral series in particular was first successfully explained by the Rutherford-Bohr quantum model of the hydrogen atom. In some cases spectral lines are well separated and distinguishable, but spectral lines can also overlap and appear to be a single transition if the density of energy states is high enough. Named series of lines include the principal, sharp, diffuse and fundamental series.
Instrument Used in Spectroscopy
There are different instruments which are used for different spectroscopy techniques. But we will discuss the general parts which are common for all the instruments.
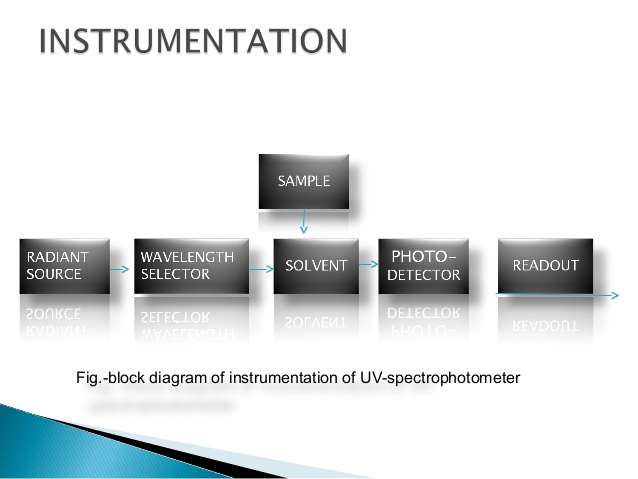
Source
The first part is Source of our energy (radiations) .The source will provide the radiations according to the technique of spectroscopy e.g for Infrared Spectroscopy, the source will provide Infrared rays continuously.
Monochromator
This is also known as wavelength selector. We know the source will provide a large range of wavelengths, but we need a particular wavelength for our sample. This will provide a particular single wavelength. Monochromator acts as a filter for other wavelengths.
Sample Holder
As the name suggests, this will hold our sample. One important function of the sample holder is that it should not interact with the sample or we can say that the sample holder should be neutral.
Detector
The detector detects the changes or the interactions of the radiations with the sample. Then send the signal to output device.
Different Types of Spectroscopy
By using only one spectroscopic technique we cannot full information about a compound. So different techniques tell different results and from these different results we can have full information about the compound.
So now we will discuss different techniques briefly
Note: We will discuss all these techniques in detail in upcoming blogs
- Infrared Spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms.
The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency or wavelength on the horizontal axis.
General Infrared Spectrum
- NMR (Nuclear Magnetic Resonance)
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds. Similarly, biochemists use NMR to identify proteins and other complex molecules. Besides identification, NMR spectroscopy provides detailed information about the structure, dynamics, reaction state, and chemical environment of molecules. The most common types of NMR are proton and carbon-13 NMR spectroscopy, but it is applicable to any kind of sample that contains nuclei possessing spin.
NMR spectra are unique, well-resolved, analytically tractable and often highly predictable for small molecules. Different functional groups are obviously distinguishable, and identical functional groups with differing neighboring substituents still give distinguishable signals.
- Raman Spectroscopy
Raman spectroscopy is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified.
Raman spectroscopy relies upon inelastic scattering of photons, known as Raman scattering. A source of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range is used, although X-rays can also be used. The laser light interacts with molecular vibrations, phonons or other excitations in the system, resulting in the energy of the laser photons being shifted up or down. The shift in energy gives information about the vibrational modes in the system. Infrared spectroscopy typically yields similar, complementary, information.
- Rotational Spectroscopy
Rotational spectroscopy is concerned with the measurement of the energies of transitions between quantized rotational states of molecules in the gas phase. The spectra of polar molecules can be measured in absorption or emission by microwave spectroscopy or by far infrared spectroscopy. The rotational spectra of non-polar molecules cannot be observed by those methods, but can be observed and measured by Raman spectroscopy. Rotational spectroscopy is sometimes referred to as pure rotational spectroscopy to distinguish it from rotational-vibrational spectroscopy where changes in rotational energy occur together with changes in vibrational energy, and also from ro-vibronic spectroscopy (or just vibronic spectroscopy) where rotational, vibrational and electronic energy changes occur simultaneously.
- Mass Spectroscopy
Mass spectrometry (MS) is an analytical technique that measures the mass-to-charge ratio of ions. The results are typically presented as a mass spectrum, a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures.
A mass spectrum is a plot of the ion signal as a function of the mass-to-charge ratio. These spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical identity or structure of molecules and other chemical compounds.

3 comments for “Spectroscopy – Legendshub Blog”